When Crystals Flow: Polymer’s Melting Point: Semicrystalline polymers are solids that are assumed to flow only above their melting temperature. In a new study published in Science Advances, Chien-Hua Tu and a research team at the Max Planck Institute for Polymer Research in Germany and the University of Ioannina Greece confined crystals within nanoscopic cylindrical pores to show the flowing nature of semicrystalline polymers below their melting point, alongside an intermediate state of viscosity to the melt and crystal states.
The capillary process was strong during the phenomenon and dragged the polymer chains into the pores without melting the crystal. The unexpected improvement in flow facilitated polymer processing conditions applicable to low temperatures, suited for use in organic electronics.
Crystalline state
About 2,500 years ago, the philosopher Heraclitus proposed that “everything flows,” and while perfect crystals at zero temperature do not flow, crystalline materials do flow under specific conditions. For example, existing research from about 100 years ago showed that the flow of cast iron in the form of flowing metal grains surrounded by a thin amorphous layer is analogous to an undercooled liquid.
Using molecular dynamics simulations, researchers have confirmed the ideas to further suggest the significance of the complex grain boundary “fluid” on plastic deformation. For instance, the inner core of the Earth is similarly proposed to retain iron in a crystalline state. Furthermore, the core of planets such as Neptune and Uranus are composed of superionic crystalline water, and flow to generate their magnetic field, which may have ultimately led to our own existence.
Crystalline materials that exhibit fluid-like mobilities are known as “superionics” and are important for energy applications. Semicrystalline polymers are solids that do not flow under normal conditions. In this work, Tu and colleagues showed how even the semicrystalline polymers underwent flow. To examine the phenomenon, they used two semicrystalline polymers; poly (ethylene oxide) and poly(ε-caprolactone) with specific molecular characteristics. The materials scientists developed self-ordered nanoporous alumina templates for the study, based on existing literature protocols.
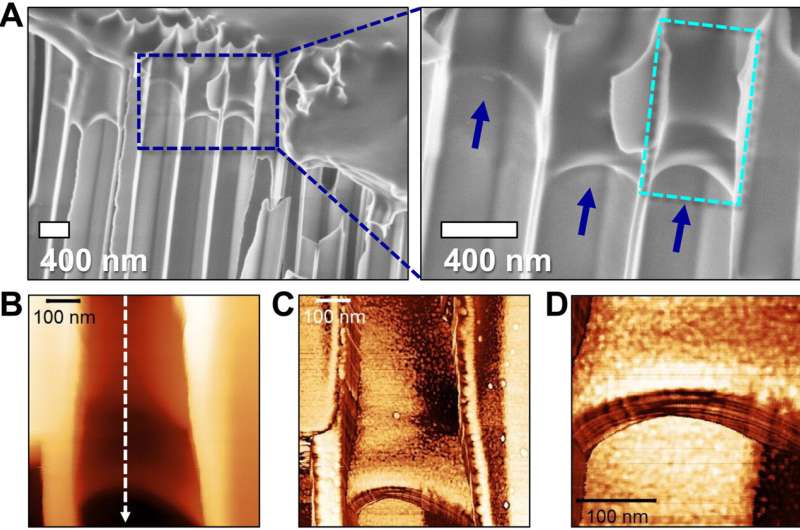
Materials characterization
The scientists examined the thermodynamics, structural and rheological properties of bulk polyethylene oxide materials. And the data confirmed the material film on the alumina template to be in a semicrystalline state. The team observed the domain spacing organization of crystalline lamellae with small-angle X-ray scattering. They used polarizing optical microscopy to study the superstructure of bulk polyethylene oxide with a film slowly cooled from melt to ambient temperature. The outcomes indicated a single spherulitic superstructure for polyethylene oxide, while the structural dynamics of poly(ε-caprolactone) synthesized with a catalyst differed.
The research team carried out a 28-day imbibition (uptake of water that leads to swelling of materials) of the two polymer materials within anodic aluminum oxide templates and observed the samples with scanning electron microscopy and atomic force microscopy to characterize them. In contrast to the relatively smooth appearance of polyethylene oxide, the poly(ε-caprolactone) materials showed abundant grain structures due to diverse morphological origins in intracrystalline diffusion. After studying the surface appearances of materials, the researchers performed nano-infrared microscopy to obtain additional images of the surface topography of the two materials. The outcomes clearly showed the semicrystalline nature of polyethylene oxide. They also addressed the possibility of the capillary force in the experimental setup to be sufficiently high to melt the crystals during flow and noted the viscosity of semicrystalline polymers to be reduced during the experiments.
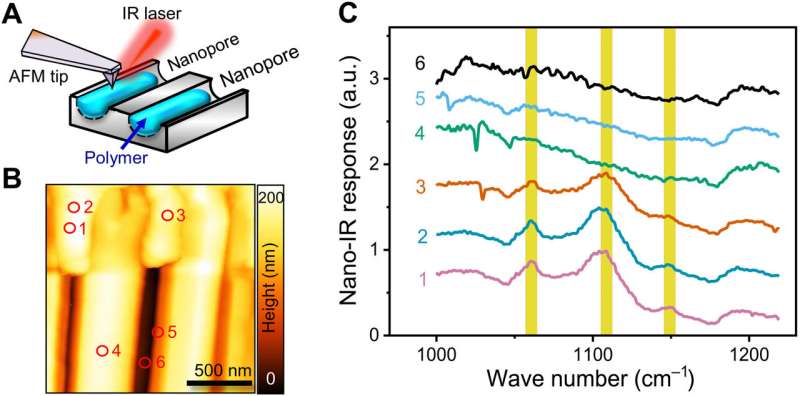
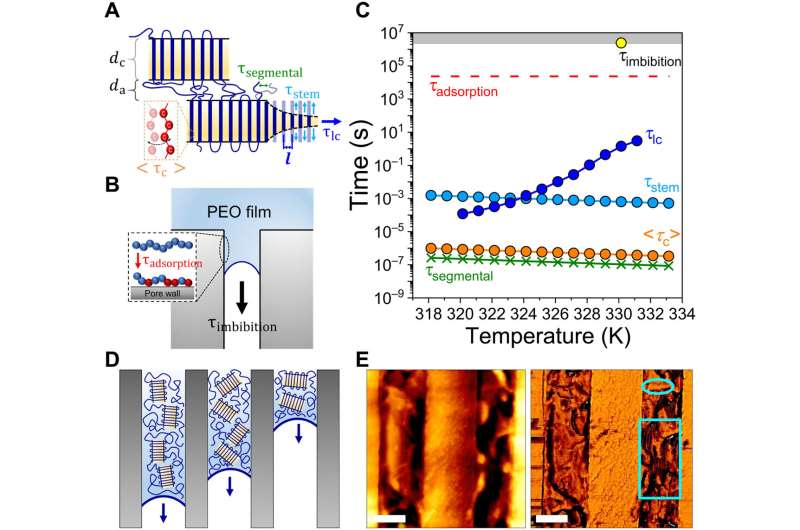
Mechanisms of imbibition
The mechanisms of fluid uptake and materials swelling known as imbibition from the semicrystalline state relied on the dynamics of its crystalline and amorphous domains. Four processes acted on the amorphous and crystalline regions; segmental relaxation governed the dynamics in the amorphous domain, whereas three other processes affected the crystalline domain to demonstrate intracrystalline chain diffusion for crystal-mobile polymers such as polyethylene oxide.
Since the imbibition of crystals also involved the diffusion of entire crystallites, Tu and the team examined the influence of the molar mass of the polymers on the process of imbibition. The outcomes showed that the molar mass regulated the imbibition speed.
Outlook
In this way, Chien-Hua Tu and colleagues used several imaging methods in materials science, such as scanning electron microscopy, atomic force microscopy, and nano-infrared results to examine how semicrystalline polymers underwent flow within nanopores made of anodic aluminum oxide via capillary action. They measured the viscoelastic behavior of the polymers with a shear rheometer, and the capillary action appeared to drive the polymer adsorption process.
While successful imbibition was a relatively slow process, the capillary force was strong enough to drag polymer crystallites into the nanopores without melting the crystals. The unexpected increase in flow while preserving the polymer crystallites applied to polymer processing at low temperatures. Such phenomenon can lead to cold flow and subsequent bonding of polymers to ceramics or metal under specific conditions to prevent polymer degradation. Such semicrystalline polymers and ferroelectric materials have a variety of applications in organic electronics to affect their electronic and physical properties.
When Crystals Flow: Polymer’s Melting Point: Original Article
Einstein-Podolsky-Rosen paradox observed in many-particle system for the first time